The Shape of Ch4 and Ccl4 Are Best Described as
The dipole moments of ccl4 chcl3 and ch4 are in the order - Get the answer to this question and access more number of related questions that are tailored for students. General Organic Biological Chemistry 1st Edition Edit edition Solutions for Chapter 4 Problem 63P.
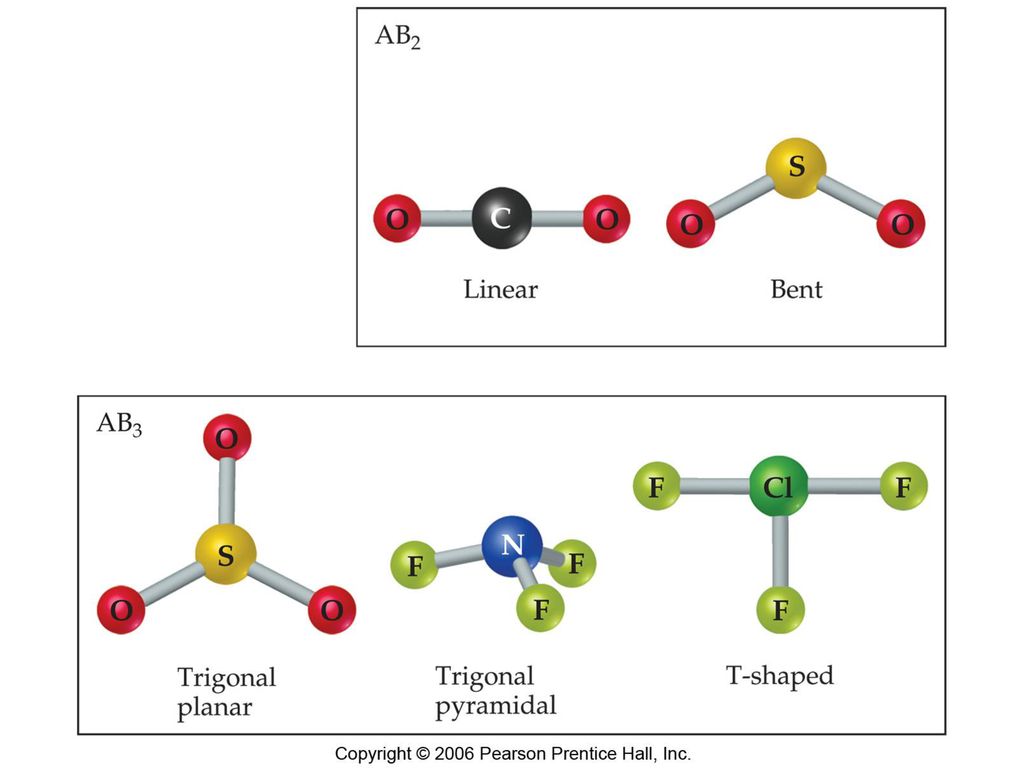
Figure Un Title Lewis Structure Caption Ccl4 Ppt Download
CH4 BECAUSE a dipole is a polar covalent bond and has asymmetrical shape CH4 is none of these The weakest Van der Waals forces exists between molecules of-C2H6 -C3H8.
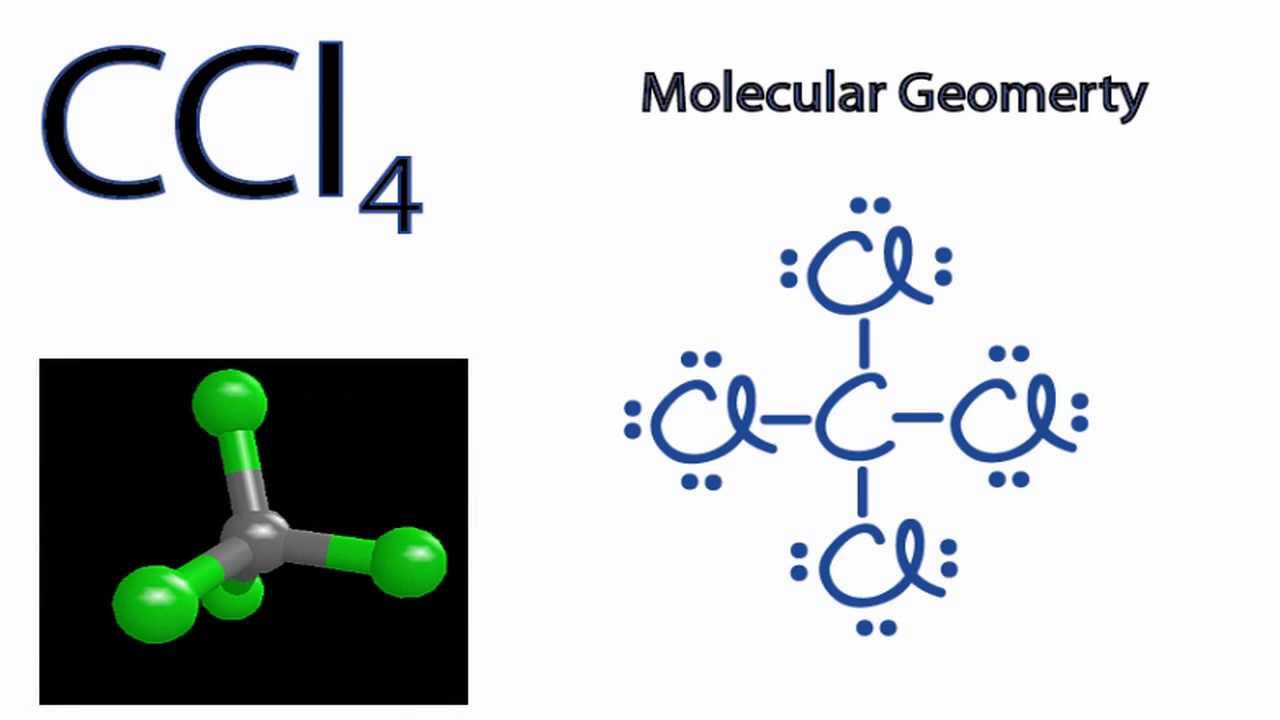
. Carbon completes its octet by forming bonds with four chlorine atoms. The molecular geometry of carbon tetrachloride CCl 4 is best described as. Draw the Lewis dot structure for CCl_4 and indicate the shape of the molecule Draw the Lewis dot structure for NH_3 and indicate the shape of the molecule Lewis dot structure.
Hybridization lowers the energy of all atomic orbitals. BF3 CH4 NH3 SF6. Biological Chemistry 1st Edition Textbook Solutions.
Hydrogen is too small an atom for its atomic orbital to overlap with a carbon atomic orbital. B What is the bond order of CO. In CCl4 the C atom is sp3 hybridised.
Which of the following is not a form of chemical bonding. E I IV. -high surface tension-high specific heat in C -solid less dense than liquid since ice floats.
Chemistry questions and answers. CCl4 MgCl2 H2O CO2. That is why the dipole moment of CCl4 is considered as 0.
Using the bond energies provided below calculate ΔH for the reactionCH4 g 4Cl2 g CCl4 g 4HCl gBond energies. In CCl4 molecule all the dipole moment is towards Cl and they are equal and opposite. C-H 413 kJmol Cl-Cl 243 kJmol C-Cl 339 kJmol H-Cl 427 kJmol.
3 bonds 1 lone pair The theory of matters conceptual foundation involved contributions from. A ground state carbon atom has two unpaired electrons. The net dipole moment of any three dipole moment is equal and opposite to 4th dipole moment so net dipole moment becomes Zero hence it is non - polar molecule but bonds are polar.
Due to this type of geometrical shape the resultant of 3 C-Cl bond moments neutralise the other one or resultant of 2 C-Cl bond moments neutralise the resultant of other two C-Cl bonds. A 1 B 15 C 2 D 25 E 3. What is the molecule count of 567g of CCl4.
Which of the following explains this phenomenon. Chemistry questions and answers. The hybridization of CCl4 is sp3 and has a tetrahedral shape.
1 For the following compounds CH4 CF4 CCL4 CBr4 what molecular shapes these molecules adopt according to VSEPR theory. Draw Lewis structures for CCl4 and C2Cl4. The shape of CH4 and CCl4 are best described as A tetrahedral B pyramidal C hexagonal D octahedral.
D are unevenly distributed through the molecule. So the structure of the molecule is Tetrahedral. What is the shape of a molecule with two bond charge clouds and two electron pair charge.
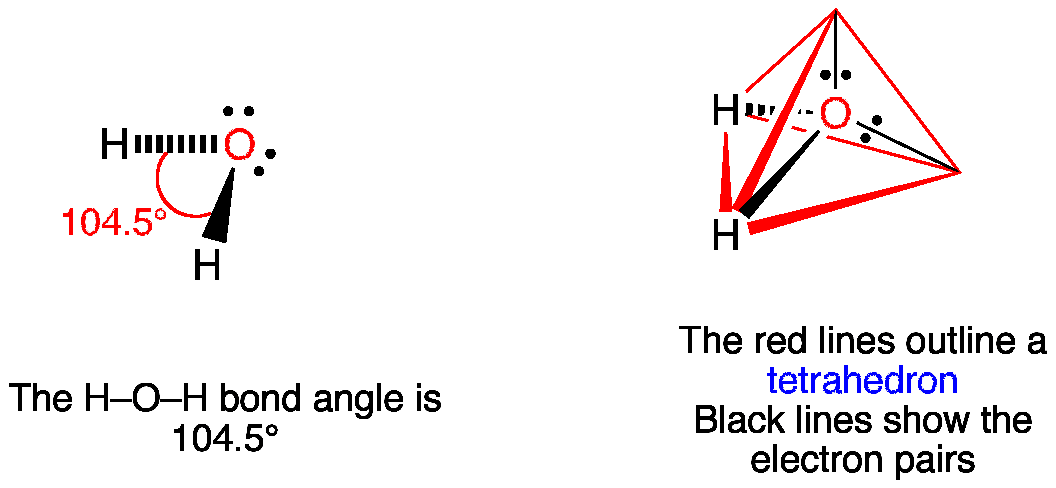
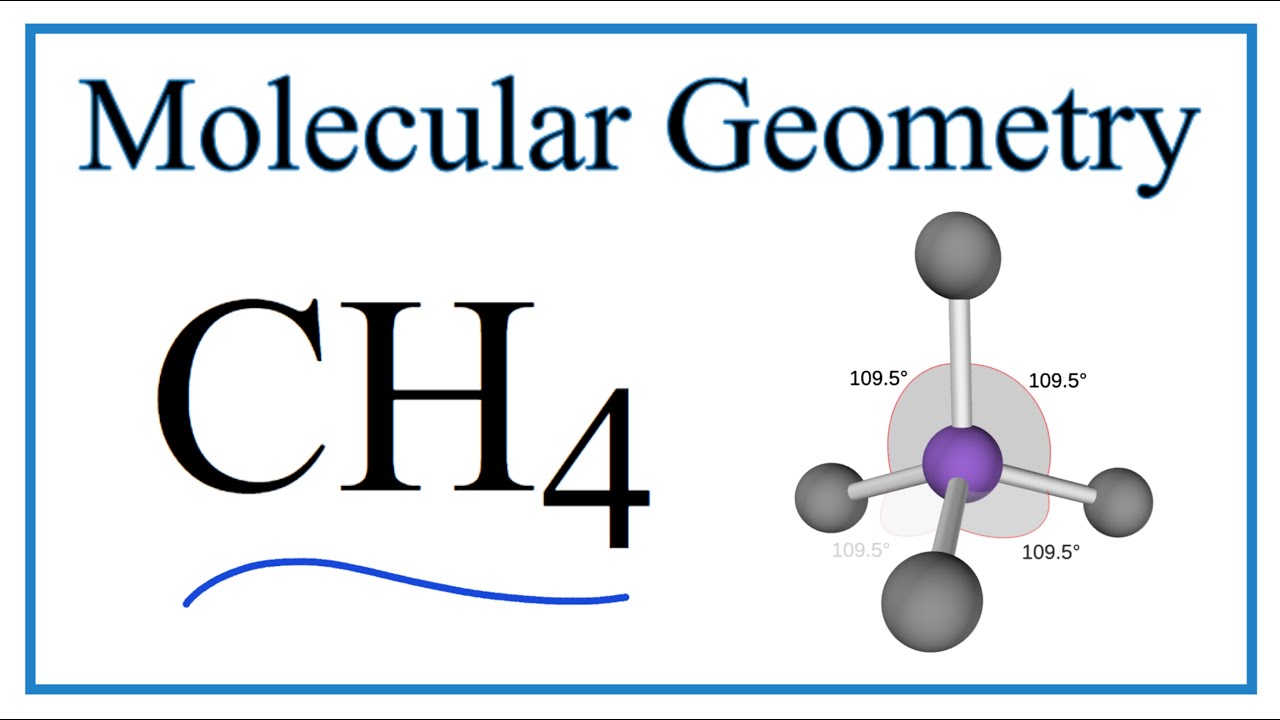
Give the molecular shape around each carbon atom. The bond angle is 1098 degrees between the lone pairs of electrons and it is nonpolar. Choose An Option That Best Describes Your Problem.
You can add the two remaining valence electrons onto O and check that. Oxygen has six valence electrons but as it tends to accept two it tends to make two bonds in covalent compounds. 2 For non-polar molecules what types of intermolecular forces do these molecules have.
Asked Sep 3 2019 in Chemistry by WilsonDyke. Which of the following is tetrahedral. C form the electron pairs in the C-H bonds of the compound.
Are they polar or non-polar molecules. The shape is a tetrahedral so the bond angles are 1095 degrees. 1 mole CCl4 153811g CCl4 6022 x 1023 molecules CCl4 567g CCl4 x 6022 x 1023.
Moreover as there exist sigma bonds only and one 2s and three 2p orbitals of the carbon produce four new hybrid orbitals the hybridization of CH4 is sp3. How Is The Amoeboid Shape Of Wbcs Helpful To Them. Hybridization raises the energy of all atomic orbitals.
2 bonds 0 lone pairs. A covalent b hydrogen. The shape of BrF3 is best described as a linear b pydramidal c trigonal planar d T-shaped.
The shape of CH4 and CCl4 are best described as CH4 g 4Cl2 g CCl4 g 4HCl g ΔH 434 kJ Methane CH4 is a gas but carbon tetrachloride CCl4 is a liquid at room conditions. A ground state carbon atom has two unpaired electrons. Answer not in Detail.
Which of the following compounds have trigonal planar molecular geometry. It is interesting to realize that irrespective. Explain why the carbon atoms in the two molecules have different shapes.
D The electrons in the delocalized molecular orbitals of benzene C6H6 A are confined between two adjacent bonding atoms. Carbon Tetrachloride is an IUPAC. How that type of intermolecular force.
What is the O-N-O bond angle in the nitrate ion. B are free to move around the six-membered ring. AlCl3 AlH3 BF3 NH3.
Which of the following is not a trigonal planar molecule. Considering atoms with no formal charge which statement best describes the valence of carbon nitrogen and oxygen. Hydrogen is almost always going to make only one bond given that it only has one valence electron.
The rest 28 electrons are non-bonding electrons. Although the four bonds C-Cl are polar because of the difference in electronegativity of Chlorine 316 and Carbon 255 CCl4 is nonpolar because the bond polarity gets canceled with each other due to the symmetrical geometrical structure tetrahedral of the CCl4 molecule. Eventually you should get that formaldehyde looks like this.
CCl4 Carbon tetrachloride is nonpolar in nature. Rank these liquids by their expected surface tension. Which of the following compounds have trigonal pyramidal molecular geometry.
The Lewis structure of the methane CH4 molecule is drawn with four single shared covalent bonds between the carbon and hydrogen atoms each.
Ccl4 Molecular Geometry Shape And Bond Angles Youtube

Figure Un Title Lewis Structure Caption Ccl4 Ppt Download

Is Hbr Polar Or Nonpolar Hydrogen Bromide Molecules Polar Ball Exercises

Ccl4 Lewis Structure Molecular Geometry Polar Or Non Polar Bond Angle

Pbr5 Lewis Structure Phosphorus Pentabromide Ap Chemistry Lewis Molecules

Ch4 Molecular Geometry Science Education And Tutorials

What Is The Vsepr Shape Of The Molecule Ch4
Ch4 Methane Molecular Geometry Bond Angles Youtube

Ch4 Molecular Geometry Shape And Bond Angles Methane Molecular Geometry Molecular Geometry
What Is The Molecular Shape For Ccl4 Quora

Shapes And Polarity Of Molecules Ppt Download

Pbr5 Lewis Structure Phosphorus Pentabromide Ap Chemistry Lewis Molecules

Let S Celebrate Conscious Discipline I Love You Rituals Conscious Discipline Consious Discipline

Bcl3 Lewis Structure Boron Trichloride Math Molecules Lewis

Figure Un Title Lewis Structure Caption Ccl4 Ppt Download
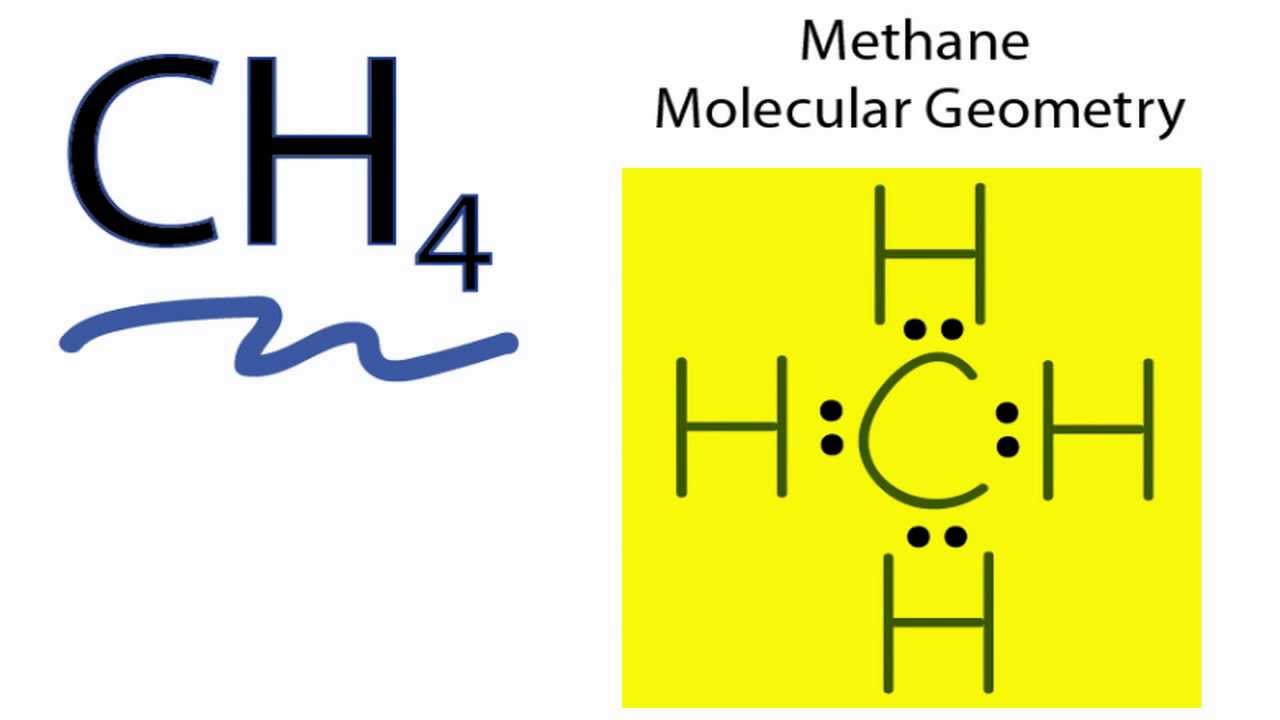
Ch4 Molecular Geometry Shape And Bond Angles Youtube

Hybridization Of Sf6 Sulfur Hexafluoride Molecules Ap Chemistry Chemical Formula
Comments
Post a Comment